Introduction
Understanding the concepts of acids, bases, and salts is crucial in the world of chemistry. As a Class 10 CBSE student, diving into these topics might seem challenging, but fear not! This comprehensive guide on Acids, Bases & Salts (Most Imp Q&A) is here to simplify the intricate world of acids, bases, and salts, ensuring a solid foundation for your science studies through the most important questions and their comprehensible answers.

Acids, Bases & Salts (Most Imp Q&A)10th Sc 2025-26
Question No. 1 to 18: MCQs
(IMP NOTE- We are giving 18 such questions for the purpose of practice. In the real exam, no. of such questions will be different).
1)
In a polluted city, water from the first rain in the monsoon season is collected in a beaker. Changes that it will have on a litmus paper
(a). Turn red litmus blue.
(b). no effect on the litmus paper.
(c)Both A and B
(d)Turn blue litmus red
Ans. Option (d).
2)
Which one is a neutralization reaction?
- 4Na + O2 → 2Na2O
- Fe + 2HCl → FeCl2 + H2
- MgO + H2O → Mg(OH)2
- HNO3 + NaOH → NaNO3 + H2O
Ans. Option (4).
3)
The following apparatus was set up in an attempt to demonstrate electrical conductivity through an electrolyte,
Which among the following statement(s) is/are correct?
The bulb will not glow because–
- The electrolyte is not acidic.
- NaOH is a strong base and furnishes ions for conduction.
- The circuit is incomplete.
- It depends upon the type of electrolytic solution.
(a) 1 and 3
(b) 2 only
(c) 2 and 4
(d) 4 only
Ans. Option (b).
4).
A boy X rubbed the yellow-colored turmeric stain on his friend’s shirt with soap. He observed that the color of the stain became:
- Pink
- Reddish brown
- Remained yellow
- White
Ans. Option (2).
5).
The yellow color of turmeric changes to red when soap solution is added to it. However, if substance P is added to turmeric, there is no change in color.
Which one is definitely true about substance P?
- P is an acid
- P is not a salt
- P is not a base
- P is a neutral substance
Ans. Option (3)
6).
Which of the following are properties of acids?
- They are bitter in taste.
- They react with metals to produce hydrogen gas.
- They are easily soluble in water.
Options
- Only P
- Only P and R
- Only Q and R
- All – P, Q, and R
Ans. Option (3).
7).
viii. Which of the following are correctly matched?
| 1. Acid + Salt | Metal + Hydrogen |
| 2. Acid + Metal Carbonate | Salt + Carbon Dioxide + Water |
| 3. Metal Oxide + Acid | Salt + Water |
Options:
- 1 and 2
- 2 and 3
- 1 and 3
- 1, 2 and 3
Ans. Option (2).
8).
Which of the following are correctly matched?
| 1. Common salt | Formed by sodium hydroxide and hydrochloric acid |
| 2. Brine | The aqueous solution of Sodium chloride |
| 3. Chlor-alkali process | Formation of Sodium chloride |
- 1 and 2
- 2 and 3
- 1 and 3
- 1, 2 and 3
Ans. Option (1).
9).
Aman took four colorless solutions P, Q, R, and S, and performed the following tests.
| Solution P | Solution Q | Solution R | Solution S | |
| With Methyl Orange | No change in color | Turns red | No change in color | No change in color |
| With Phenolphthalein | No change in color | No change in color | No change in color | Turns pink |
| With Red litmus | No change in color | No change in color | No change in color | Turns litmus blue |
| With Blue litmus | No change in color | Turns litmus red | No change in color | No change in color |
What is the definite conclusion that Aman can get?
- Both P and S are salt solutions
- Both Q and S are basic solutions
- Q and R Both are salt solutions
- P and R Both are neutral solutions.
Ans. Option (4).
10).
In a polluted city, water from the first rain in the monsoon season is collected in a beaker. Changes will it have on a litmus paper?
- Turn red litmus blue.
- No effect on the litmus paper.
- Both A and B
- Turn blue litmus red
Ans. Option (4).
(CONTINUED AFTER THE REFERENCE BOOK LIST)
Best Reference Book for CBSE Science 10




11).
Preeti has to arrange the following in decreasing order of hydroxide ion concentration.
Wine (pH 4.0), Milk of magnesia (pH 10.5), Blood (pH 7.4)
Which of the following arrangements is correct?
- wine, milk of magnesia, blood.
- blood, milk of magnesia, wine.
- milk of magnesia, blood, wine
- wine, blood, milk of magnesia.
Ans. Option (3).
12).
Sunita added a drop each of diluted acetic acid and diluted hydrochloric acid on pH paper and compared the colors. Which one is the correct conclusion?
- pH of acetic acid is greater than that of hydrochloric acid.
- pH of acetic acid is smaller than that of hydrochloric acid.
- in an aqueous solution, acetic acid dissociates completely.
- Acetic acid is a strong acid.
Ans. Option (1).
13).
The graph given below depicts a neutralization reaction (acid + base → salt + water). If we add an excess of acid to an alkali,
The pH of a solution changes
Choose the letter denoting the area of the graph where both acid and salt are present?
- A
- B
- C
- D
Ans. Option (4).
14).
There is a solution of a base with pH 12.1,
Which one can be done to decrease its pH?
- Adding distilled water to it.
- Adding a solution of a different base with pH 8.7
- By adding a few drops of an acid with an unknown pH
Options
- Only 1
- Only 3
- Only 1 and 2
- Any of 1, 2, and 3
Ans. Option (4)
15).
There are certain activities that cause the soil and water resources in that area to become acidic. The land has to be treated to enable plants to grow once again when these activities are stopped,
What should be added to the land to decrease the acidity permanently and allow plants to grow once again?
- Water which is neutral.
- Calcium oxide which is basic.
- Sodium chloride which is neutral.
- Dilute hydrochloric acid solution.
Ans. Option (2).
16).
Which of the following are correctly matched?
| 1. Plants and animals | pH range is 7.0 to 7.8 |
| 2. Acid rain | pH is 7.6 |
| 3. Tooth Decay | pH less than 5.5 |
- 1 and 2
- 2 and 3
- 1 and 3
- 1, 2, and 3
Ans. Option (3). (pH of acid rain is less than 5.6)
17).
Match the following-
| Column A | Column B |
| A. Bleaching powder | i. Preparation of glass |
| B. Baking Soda | ii. Production of H2 and Cl2 |
| C. Washing Soda | iii. Decolorization |
| D. Sodium Chloride | iv. Antacid |
- A-ii, B-I, C-iv, D-iii
- A-iii, B-ii, C-iv, D-i
- A-iii, B-iv, C-i, D-ii
- A-ii, B-iv, C-i, D-iii
Ans. Option (3).
18).
Salts that does not contain water of crystallization?
- Blue vitriol
- Baking soda
- Washing soda
- Gypsum
Ans. Option (2).
Q.No.19 to 28
(Assertion and Reasoning type questions)
Acids, Bases & Salts (Most Imp Q&A)
(IMP NOTE- We are giving 10 such questions for the purpose of practice. In the real exam, no. of such questions will be different).

(a) Both Assertion and Reasoning are true, and Reasoning is the correct explanation of Assertion.
(b) Both Assertion and Reasoning are true, but Reasoning is not the correct explanation of Assertion.
(C) Assertion is true, but Reasoning is false.
(d) Both Assertion and Reasoning are false.
19). Assertion: Litmus paper turns blue when dipped in a solution containing a base.
Reasoning: Litmus paper is a natural indicator that changes color in the presence of acidic or basic solutions.
Answer: a. Both Assertion and Reasoning are true, and Reasoning is the (correct explanation of Assertion.
20). Assertion: Dilution of an acid decreases its acidity.
Reasoning: Dilution increases the concentration of hydrogen ions (H+) in the solution, reducing the acidity of the solution.
Answer: a. Both Assertion and Reasoning are true, and Reasoning is the correct explanation of Assertion.
21).Assertion: Bases are proton acceptors.
Reasoning: Bases react with acids by accepting protons (H+), forming water and a salt.
Answer: a. Both Assertion and Reasoning are true, and Reasoning is the correct explanation of Assertion.
22).Assertion: Sodium hydroxide is a strong base.
Reasoning: Strong bases ionize completely in aqueous solutions to produce hydroxide ions (OH-).
Answer: a. Both Assertion and Reasoning are true, and Reasoning is the correct explanation of Assertion.
23). Assertion: The pH scale is logarithmic.
Reasoning: The pH of a solution is defined as the negative logarithm (base 10) of the concentration of hydrogen ions in the solution.
Answer: a. Both Assertion and Reasoning are true, and Reasoning is the correct explanation of Assertion.
24). Assertion: Ammonia (NH₃) is a weak base.
Reasoning: Weak bases partially ionize in aqueous solutions, resulting in fewer hydroxide ions (OH-) compared to strong bases.
Answer: a. Both Assertion and Reasoning are true, and Reasoning is the correct explanation of Assertion.
25). Assertion: Hydrochloric acid (HCl) is a strong acid.
Reasoning: Strong acids ionize completely in water, releasing a high concentration of hydrogen ions (H+).
Answer: a. Both Assertion and Reasoning are true, and Reasoning is the correct explanation of Assertion.
26). Assertion: Acid rain can damage buildings and statues made of limestone.
Reasoning: Limestone is primarily composed of calcium carbonate, which reacts with acid rain (containing sulfuric and nitric acids) to form soluble salts.
Answer: a. Both Assertion and Reasoning are true, and Reasoning is the correct explanation of Assertion.
27). Assertion: Acids have a sour taste, while bases have a bitter taste.
Reasoning: The taste of acids and bases is a characteristic property due to the presence of hydrogen ions (H+) in acids and hydroxide ions (OH-) in bases.
Answer: a. Both Assertion and Reasoning are true, and Reasoning is the correct explanation of Assertion.
28). Assertion: When an acid reacts with a metal, hydrogen gas is evolved.
Reasoning: The reaction between an acid and a metal involves the displacement of hydrogen ions from the acid by the metal, resulting in the formation of a salt and the liberation of hydrogen gas
Answer: a. Both Assertion and Reasoning are true, and Reasoning is the correct explanation of Assertion.

Q. No. 29-44 (SA & LA Type Questions)
Acids, Bases & Salts (Most Imp Q&A)
29).
Name the compound of calcium that is used for the plastering of fractured bones. With the help of a chemical equation show how is it prepared and what special precautions should be taken during the preparation of this compound.
Ans. Plaster of Paris (CaSO4.1⁄2H2O)
On heating at a temperature of 373 K, gypsum loses water molecules and gives calcium sulphate hemihydrate
CaSO4.2H2O (Heated at 373 K)→ CaSO4.1⁄2H2O + 11⁄2H2O
Precaution: Gypsum should not be heated above 373 K because it will form CaSO4 if heated above 373 k.
30).
(i) A salt is used to remove the permanent hardness of the water. Give the chemical name and chemical formula of the salt.
(ii) What is olfactory indicators?
Ans (a). Sodium Carbonate Decahydrate (Washing Soda)
(b). Na2CO3.10H2O
Ans (ii). There are some indicators that show a change in their odor in the presence of acids or bases. Such indicators are called olfactory indicators. These indicators are very useful for visually challenged people because such people cannot use other indicators.
31).
(i). Mr. X found that the Plaster of Paris, which he stored in a container, had become very hard and lost its binding nature. What is the reason for this? Write a chemical equation also to represent the reaction taking place.
ii. Write one use of Plaster of Paris other than plastering the walls.
Ans.
(i)
It converted into Gypsum because of the moisture present in the atmosphere,
CaSO4.1⁄2H2O + 11⁄2H2O → CaSO4.2H2O (Gypsum)
(ii).
Uses of Plaster of Paris:
- Making toys, dolls, or statues
- Fixing broken limbs
- Making decorative materials.
32).
How will you prove that chemical change has taken place in the given reaction
Dil. HCl is added to Zn granules.”?
Explain your answer with the help of examples.
Ans.
- Bubbles of gas/ Evolution of gas
- Color change ( silvery gray to black)
- Change in temperature
33).
What is water of crystallization? How can we show that copper sulphate crystals contain water of crystallization?
Ans.
Water of crystallization are the molecules of waterattached with a crystalline substance.
On heating, hydrated copper sulphate changes its colour from blue to dirty white and water droplets are formed.
CuSO4.5H2O → CuSO4 + 5H2O.
If we add a little water to anhydrous CuSO4, we get the blue color again.
CuSO4 + 5H2O → CuSO4.5H2O
34).
Given below is the schematic diagram for the preparation of hydrogen gas .What would happen if the following changes are made?
(a). If the same amount of zinc dust is taken in the test tube in place of Zn granules.
(b). If we take dilute hydrochloric acid in place of dilute sulphuric acid.
(c). If Cu turnings are taken in place of Zn.
(d). In place of sulphuric acid , if we take sodium hydroxide and the tube is heated.
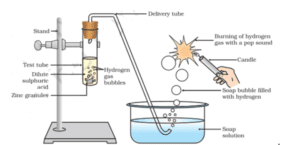
Ans.
(a) In that case, hydrogen gas will evolve with a greater speed because dust particles are smaller in comparison to granules.
(b) The amount of gas evolved is almost the same.(Both are strong acids).
(c) Hydrogen gas is not evolved. No reaction will take place because Cu is below hydrogen in the reactivity series, and it cannot displace hydrogen from acids.
(d) Hydrogen gas will be evolved, if sodium hydroxide is taken.
Zn + 2NaOH → Na2ZnO2 (Sodium Zincate) + H2.
35).
(i). A calcium compound is used as a disinfectant. Mention the name and chemical formula of the compound? How is this compound manufactured?
(ii). What are the products obtained when sodium hydrogen carbonate is heated? Show it by writing the chemical equation for the reaction.
Ans.
(i). Name: Bleaching Powder
Chemical formula: CaOCl2
Preparation: Bleaching powder is formed on passing chlorine gas through slaked lime.
Ca(OH)2 + Cl2 → CaOCl2 + H2O
(ii) Sodium carbonate, carbon dioxide, and water.
2NaHCO3 → Na2CO3 + CO2 + H2O
36).
A colorless, odorless gas X was evolved which turned lime water milky when Mr. Mehta took sample A and added dilute hydrochloric acid to it. Write the names of sample A and the gas X evolved. Also write a chemical equation to explain the reaction between sample A and Hydrochloric acid. The gas X turn lime water milky. Why?
Ans.
Sample A= metal carbonate and
The gas X = carbon dioxide.
Na2CO3 + 2HCl → 2NaCl + CO2 + H2O
When CO2 reacts with lime water, an insoluble substance named calcium carbonate is formed which is white in color . So, the solution appears milky.
Ca(OH)2 + CO2 → CaCO3 + H2O
37).
Write the chemical equation to represent the change-Chlorine gas is prepared using electrolysis of brine solution. Also Identify the other products formed in the process and give one application of each.
Ans.
2NaCl (aq) + 2H2O (l) → 2NaOH (aq) + Cl2 (g) + H2 (g)
Other products formed during the electrolysis of brine solution are:
- Sodium hydroxide/ NaOH/ Caustic soda
- Hydrogen
Uses:
1. Sodium hydroxide/NaOH/Caustic soda
- Degreasing of metals
- Preparation of soaps and detergents
- Papermaking
- Artificial fibers
38).
Write the difference between acidic and basic salts with an example.
Ans.
By the neutralization reaction between a strong acid and a weak base, acidic salts are formed.
For example, Ammonium chloride.
However, basic salts are formed by the neutralization reaction between a strong base and a weak acid.
For example, Sodium acetate.
Acids, Bases & Salts (Most Imp Q&A)
39).
A metal carbonate X reacts with acid to give a gas which when passed through a solution Y gives the carbonate back. However, if the gas G(obtained at the anode during electrolysis of brine) is passed on dry Y, it gives a compound Z(used for disinfecting drinking water). Identify X, Y, G, and Z. Also write the chemical reactions involved.
Ans. During the electrolysis of brine,the gas G evolved at the anode is chlorine.
When chlorine gas is passed through dry Ca(OH)2 (Y), it produces bleaching powder (CaOCl2) (Z) used for disinfecting drinking water.
Ca(OH)2 + Cl2 → CaOCl2 + H2O
Since Y and Z are calcium salts, therefore X is also a calcium salt and it is calcium carbonate.
CaCO3 + 2HCl → CaCl2 + CO2 + H2O
Ca(OH)2 + CO2 → CaCO3 + H2O
40).
(a). With the help of the experiment given below, Write the names of the gases evolved at the anode and cathode.
(b). Name the process that occurs. Why is it called so?
(c). Show the reaction of the process with the help of a chemical equation.
(d). For each of the products of the above process, mention one use and give examples of other compounds that can be formed using Sodium chloride as a raw material (reactant).
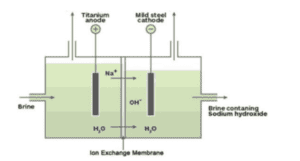
Ans.
(a). Anode: Chlorine; Cathode: Hydrogen.
(b). This is Chlor Alkali process because the products obtained are alkali, chlorine gas, and hydrogen gas.
(c). 2NaCl + 2H2O + electric current → 2NaOH + Cl2 + H2
(d). Sodium hydroxide- making of soaps and detergents, paper making.
Chlorine- as a disinfectant (for water treatment, in swimming pools),
as an oxidizing agent.
Hydrogen- as fuel, making ammonia for fertilizers.
Baking soda and Washing soda are prepared by using sodium chloride..
41).
Give suitable reasons for the following statements:
i. Distilled water does not conduct electricity but rainwater does.
ii. When we overeat, we feel a burning sensation in the stomach.
iii. When rubbed with lemon, a tarnished copper vessel regains its shine
iv. On exposure to air, the crystals of washing soda change to white powder
v. An aqueous solution of sodium carbonate is basic but an aqueous solution of sodium chloride is neutral
vi. Dilute hydrochloric acid turns blue litmus red but dry hydrogen chloride gas does not.
vii. A milkman usually adds a very small amount of baking soda to fresh milk during the summer season.
viii. Ammonia is a base but it does not contain a hydroxyl group.
Ans.
i. Distilled water is a pure form of water. It does not dissociate into ions because it is neither acidic nor basic in nature. But, conduction of electricity requires free ions. Rainwater is an impure form of water and it contains many ionic substances. In fact,these ions are responsible for the electrical conductivity of rainwater.
ii. When we overeat, we feel a burning sensation in the stomach because too much HCl is produced in our stomach. This causes the burning sensation.
iii. Because of the formation of basic copper carbonate, Copper vessels are tarnished. Basic copper carbonate gets neutralized and the copper vessel regains its shine when rubbed with lemon..
iv. Washing soda (Na2CO3.10H2O) when exposed to air, it loses 10 molecules of water and changes to white powder.
v. An aqueous solution of sodium chloride is neutral because Sodium chloride is a salt of strong acid HCl and a strong base NaOH.
An aqueous solution of sodium carbonate is basic because Sodium carbonate is a salt of weak acid H2CO3 and strong base NaOH.
NaCl + H2O → NaOH + HCl
Na2CO3 + H2O → NaOH + H2CO3
vi. Dry HCl gas does not dissociate into ions (H3O+), so it has no effect on the litmus. Hydrochloric acid forms ions (H3O+), so it turns blue litmus red.
vii. A milkman usually adds a very small amount of baking soda to fresh milk during the summer season because baking soda prevents the formation of lactic acid when milk turns sour.
viii. Ammonia (NH3) dissolves in H2O forming NH4OH, therefore it acts as base:
NH3 + H2O → NH4OH → NH4+ + OH-
42).
Write the source for the following:-
i. Lactic acid
ii. Methanoic acid
iii. Tartaric acid
iv. Citric acid
v. Oxalic acid
vi. Acetic acid
Ans.
i. Sour milk (curd)
ii. Ant sting/nettle sting
iii. Tamarind
iv. Citrus fruits like lemon, orange
v. Tomato/Spinach
vi. Vinegar
43).
(i). What is the chemical name of the compound present in tooth enamel? What is the nature of the compound?
(ii). Explain why sweet tooth leads to tooth decay. Explain the role of toothpaste in preventing cavities?
Ans.
i. Chemical Name: Calcium Phosphate
Nature: Basic
ii. Tooth decay is caused by the action of bacteria on food particles remaining in the mouth. Sweet tooth leads to growth of harmful bacteria. So, the pH of the mouth falls below 5.5 and the tooth enamel dissolves. This results in cavities. Toothpaste neutralizes the excess acid produced in the mouth because toothpaste is generally basic. In this way, they prevent tooth decay.
44).
When a honeybee stings, it causes pain and irritation? How can we get relief from the discomfort?
Ans.
When The bee stings anyone, it leaves an acid called formic acid in the body. Sodium carbonate is a mild base and neutralizes the effect of formic acid. So, relief can be obtained by rubbing a mild base such as toothpaste or sodium bicarbonate (baking soda) on the affected area.
FAQs (1-8): Acids, Bases & Salts (Most Imp Q&A)
1). What is the pH scale, and how does it measure acidity or basicity?
Answer: The pH scale is a logarithmic scale that measures the acidity or basicity of a solution. It ranges from 0 to 14, with 7 being neutral. If a solution has a pH less than 7 , they are acidic and those with a pH greater than 7 are basic. The lower the pH, the more acidic the solution, and the higher the pH, the more basic it is.
2). Can you explain the process of neutralization?
Answer: Neutralization is a chemical reaction between an acid and a base, resulting in the formation of water and a salt. The hydrogen ions (H+) from the acid combine with the hydroxide ions (OH-) from the base to form water, and the remaining ions combine to form a salt.
3). How does the taste of acids and bases differ?
Answer: Acids generally have a sour taste, while bases have a bitter taste. This characteristic taste is due to the presence of specific ions – hydrogen ions (H+) in acids and hydroxide ions (OH-) in bases.
4). Why does litmus paper change color in the presence of acids or bases?
Answer: Litmus paper is a natural indicator that changes color based on the pH of a solution. It turns red in acidic solutions (pH less than 7) and blue in basic solutions (pH greater than 7), providing a quick visual indication of the nature of the substance.
5). How does dilution affect the acidity of an acid?
Answer: Dilution of an acid decreases its acidity. When an acid is diluted, the concentration of hydrogen ions (H+) in the solution decreases, leading to a reduction in acidity.
6). What happens when a metal reacts with an acid?
Answer: When a metal reacts with an acid, hydrogen gas is evolved. The metal displaces the hydrogen ions (H+) from the acid, forming a metal salt and liberating hydrogen gas.
7). Why is sodium hydroxide considered a strong base?
Answer: Sodium hydroxide is considered a strong base because it ionizes completely in aqueous solutions, producing a high concentration of hydroxide ions (OH-).
8). Can you explain the significance of the pH scale in everyday life?
Answer: The pH scale is crucial in various aspects of daily life. For instance, it helps in determining the acidity or basicity of beverages, maintaining the pH balance in swimming pools, and understanding the environmental impact of acid rain on structures and ecosystems.
Also Read-
https://pcmconceptclear.com/best-book-for-science-class-10-cbse/
https://pcmconceptclear.com/science-class-10-control-and-coordination/
https://pcmconceptclear.com/science-class-10-light-reflection-and-refraction/
https://pcmconceptclear.com/science-class-10-life-processes/
https://pcmconceptclear.com/science-class-10-acids-bases-and-salts/
https://pcmconceptclear.com/class-10-chemical-reactions-and-equations/
https://pcmconceptclear.com/chemical-reactions-and-equations-class-10-science

Признаки истинности в слове
истина это в обществознании [url=http://www.koah.ru/koret/74.htm/]http://www.koah.ru/koret/74.htm/[/url].
Thanks
Really great!
Thanks.
Фулфилмент Москва: доступные цены и прозрачные условия без скрытых платежей
фулфилмент москва цены [url=https://www.fulfilment-moskva77.ru/]https://www.fulfilment-moskva77.ru/[/url] .
Thanks.