INTRODUCTION
Before Rutherfords Nuclear Model of Atom, the concept of the atom had undergone a long evolution. Ancient Greek philosophers such as Democritus and Leucippus speculated about the existence of indivisible particles called atoms, although their ideas were more philosophical than scientific. In the 19th century, John Dalton formulated the first modern atomic theory, proposing that elements were composed of tiny, indivisible particles called atoms, each with its own unique properties. Dalton’s theory provided a framework for understanding chemical reactions and the composition of matter.
Background of Rutherfords Nuclear Model of Atom
Thomson’s experiments with cathode rays led to the discovery of the electron ( a negatively charged subatomic particle), later in the late 19th century. Thomson proposed the “plum pudding” model of the atom, where electrons were embedded in a positively charged sphere, akin to raisins in a pudding.
However, this model faced challenges when Ernest Rutherford, along with his colleagues Hans Geiger and Ernest Marsden, conducted the famous gold foil experiment in 1909. Their results defied expectations, showing that most of the alpha particles passed straight through the gold foil, but some were deflected at large angles, and a few even bounced back.
These unexpected findings led Rutherford to propose his nuclear model of the atom, suggesting that the atom consisted mostly of empty space with a dense, positively charged nucleus at its center, where most of the atom’s mass was concentrated. Electrons orbited the nucleus at a distance, much like planets orbiting the sun.
Rutherfords Nuclear Model of Atom revolutionized atomic theory, laying the groundwork for further discoveries in nuclear physics and quantum mechanics. It marked a significant shift from the earlier plum pudding model and paved the way for our modern understanding of atomic structure and behavior.
Introduction to Ernest Rutherford and his contributions to atomic theory
Ernest Rutherford, born in New Zealand in 1871, was a pioneering physicist whose contributions reshaped our understanding of the atom and laid the foundation for modern nuclear physics. His remarkable career spanned several continents, and he is widely regarded as one of the most influential scientists of the 20th century.
Rutherford’s journey into the realm of atomic theory began with his education in New Zealand and continued with his research at the University of Cambridge under the mentorship of J.J. Thomson, the discoverer of the electron. It was during this time that Rutherford conducted his groundbreaking experiments that would revolutionize our understanding of atomic structure.
One of Rutherford’s most famous experiments, conducted in collaboration with Hans Geiger and Ernest Marsden in 1909, involved bombarding a thin gold foil with alpha particles. The unexpected results of this experiment led Rutherford to propose his revolutionary nuclear model of the atom. He hypothesized that atoms consisted mostly of empty space, with a dense, positively charged nucleus at the center, surrounded by orbiting electrons.
This nuclear model of the atom represented a significant departure from previous theories, such as J.J. Thomson’s plum pudding model. Rutherford’s model provided a more accurate depiction of atomic structure, explaining the results of his gold foil experiment and paving the way for further advancements in nuclear physics.
In addition to his work on atomic theory, Rutherford made significant contributions to the field of radioactivity, identifying and characterizing several radioactive isotopes and coining the terms “alpha,” “beta,” and “gamma” to describe different types of radiation.
Throughout his career, Rutherford received numerous honors and awards, including the Nobel Prize in Chemistry in 1908 for his investigations into the disintegration of the elements and the chemistry of radioactive substances. His legacy continues to inspire generations of scientists, and his contributions to atomic theory remain foundational to our understanding of the universe at its most fundamental level.
Previous experiments leading up to the discovery of Rutherfords Nuclear Model of Atom
Before Ernest Rutherford’s groundbreaking experiments, the journey to understanding the structure of the atom was marked by incremental discoveries and experimental evidence. One of the earliest significant contributions came from the work of English chemist John Dalton in the early 19th century. Dalton proposed the first modern atomic theory, suggesting that elements were composed of indivisible particles called atoms, each with its own unique properties.
Building upon Dalton’s ideas, chemists and physicists began to explore the nature of these atoms further. In the late 19th century, the discovery of cathode rays by William Crookes and the subsequent experiments of J.J. Thomson paved the way for a deeper understanding of atomic structure. Thomson’s experiments with cathode rays led to the identification of the electron, a negatively charged subatomic particle.
Thomson proposed the “plum pudding” model of the atom, in which electrons were embedded in a positively charged sphere, much like raisins in a pudding. While this model provided a framework for understanding the presence of electrons within the atom, it did not fully explain the distribution of positive charge.
It was against this backdrop of evolving atomic theory that Ernest Rutherford conducted his groundbreaking experiments. In 1909, Rutherford, along with his collaborators Hans Geiger and Ernest Marsden, conducted the gold foil experiment. This experiment involved bombarding a thin sheet of gold foil with alpha particles, which are positively charged particles emitted by certain radioactive elements.
The results of the gold foil experiment were unexpected and defied the predictions of Thomson’s plum pudding model. Most of the alpha particles passed through the foil undeflected, some were deflected at large angles, and a few bounced back. This led Rutherford to propose his revolutionary nuclear model of the atom, suggesting that atoms consisted mostly of empty space, with a dense, positively charged nucleus at the center.
The experiments leading up to Rutherford’s discovery of the nuclear model represented significant milestones in the journey to understanding atomic structure. They laid the groundwork for Rutherford’s groundbreaking work and provided the experimental evidence needed to revolutionize our understanding of the atom.
Description of Rutherfords Nuclear Model of Atom
In 1909, Ernest Rutherford, along with his collaborators Hans Geiger and Ernest Marsden, conducted a groundbreaking experiment that would fundamentally change our understanding of atomic structure. This experiment, known as the gold foil experiment, involved bombarding a thin sheet of gold foil with alpha particles, which are positively charged particles emitted by certain radioactive elements.
The setup of the experiment was relatively simple yet ingenious. A narrow beam of alpha particles was directed at a thin foil of gold, which was only a few atoms thick. Surrounding the gold foil was a fluorescent screen coated with zinc sulfide, which would emit a flash of light when struck by the alpha particles.
The expected outcome, based on the prevailing atomic model proposed by J.J. Thomson, was that the alpha particles would pass straight through the gold foil with minimal deflection. According to Thomson’s model, the atom resembled a diffuse cloud of positive charge with negatively charged electrons embedded within it, akin to raisins in a pudding.
However, the results of the gold foil experiment were not what Rutherford and his colleagues had anticipated. While the majority of the alpha particles did indeed pass through the foil undeflected, a small fraction of them were deflected at large angles, and a few even bounced back .
This unexpected outcome was perplexing and challenged the prevailing atomic model of the time. If Thomson’s model were correct, the positively charged alpha particles should have passed through the gold foil with little to no deflection.
The significant deflections observed by Rutherford suggested that the positive charge within the atom was not uniformly distributed but rather concentrated in a small, dense nucleus at the center.
Rutherford’s interpretation of the results led to the proposal of his revolutionary nuclear model of the atom. He hypothesized that atoms consisted mostly of empty space, with a dense, positively charged nucleus at the center, where most of the atom’s mass was concentrated. Electrons orbited the nucleus at a distance, much like planets orbiting the sun.
The gold foil experiment was a pivotal moment in the history of science, as it provided experimental evidence for the existence of the atomic nucleus and paved the way for our modern understanding of atomic structure. Rutherford’s bold interpretation of the results reshaped our understanding of the atom and laid the foundation for further advancements in nuclear physics.
Best Reference Book for CBSE Chemistry11
Click the Amazon Link To Buy/Check – https://amzn.to/4kPzwyi
Pradeep’s New Course Chemistry for Class 11 (Vol. 1 & 2) Examination 2025-26

Explanation of the gold foil experiment setup which led to Rutherfords Nuclear Model of Atom
The gold foil experiment conducted by Ernest Rutherford in 1909 was a pivotal moment in the history of atomic physics. Its setup was meticulously crafted to probe the structure of the atom and challenge prevailing scientific theories of the time.
The apparatus for the experiment was relatively straightforward yet ingeniously designed. It consisted of a source emitting alpha particles, which are positively charged particles produced by the decay of radioactive elements. These alpha particles were directed towards a thin foil of gold, chosen for its high malleability and ability to be hammered into an exceedingly thin layer, just a few atoms thick.
Surrounding the gold foil was a screen coated with a fluorescent material, such as zinc sulfide. This screen acted as a detector, capable of emitting light when struck by alpha particles. By placing this screen behind the gold foil, the researchers could observe and measure the deflections of the alpha particles as they passed through the foil.
To ensure a controlled and focused beam of alpha particles, a collimator was used. This device consisted of a narrow slit through which the alpha particles could pass, helping to narrow their trajectory and minimize scattering.
The entire experimental setup was housed within a vacuum chamber to eliminate interference from air molecules and other contaminants. This ensured that the alpha particles could travel through the chamber without being deflected or absorbed by extraneous particles, thereby maintaining the integrity of the experiment.
With the apparatus assembled and the experimental conditions optimized, Rutherford and his collaborators commenced bombarding the gold foil with alpha particles. What they observed was unexpected and groundbreaking: while most alpha particles passed through the foil with little to no deflection, some were significantly deflected, and a few even bounced back in the direction from which they came.
These surprising results challenged the prevailing atomic model of the time, which proposed that atoms were composed of a diffuse cloud of positive charge with embedded electrons. Instead, Rutherford’s observations suggested that the positive charge within the atom was concentrated in a small, dense nucleus at its center.
In conclusion, the gold foil experiment’s setup was meticulously designed to explore the structure of the atom and challenge existing scientific theories. Its unexpected results paved the way for the development of Rutherford’s nuclear model and revolutionized our understanding of atomic physics.

Source: Pradeep
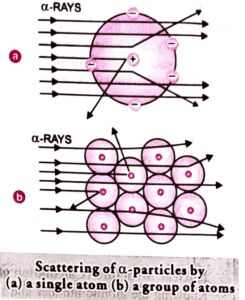
Source: Pradeep
Description of the key components of Rutherfords Nuclear Model of Atom
1. Nucleus:
-
- Central, dense region at the heart of the atom.
- Contains positively charged protons and neutral neutrons.
- Contains the majority of the atom’s mass.
- Electrons:
- Negatively charged particles that orbit the nucleus.
- Occupy orbitals or energy levels around the nucleus.
- Maintain stability by balancing their attraction to the positively charged nucleus with their repulsion from other electrons.
- Empty Space:
- Most of the atom is empty space, with electrons occupying a relatively small volume around the nucleus.
- Accounts for the observation that most alpha particles passed through the gold foil experiment undeflected.
- Planetary Model Analogy:
- Rutherford’s model often compared to a miniature solar system, with the nucleus acting as the sun and the electrons as planets orbiting around it.
- Electrons orbit the nucleus in fixed paths or orbits, analogous to the way planets orbit the sun.
- Relative Sizes and Masses:
- The nucleus is extremely small compared to the overall size of the atom.
- The mass of the nucleus is much greater than the combined mass of the electrons.
- Positive Charge Concentration:
- The positive charge of the atom is concentrated within the nucleus.
- This accounts for the strong repulsion observed in the gold foil experiment when alpha particles approached the positively charged nucleus at close range.
- Stability and Energy Levels:
- Electrons occupy specific energy levels or shells around the nucleus.
- Electrons which are closer to the nucleus have lower energy levels, while those farther away have higher energy levels.
- Stability is maintained as electrons fill these energy levels according to the aufbau principle, Pauli exclusion principle, and Hund’s rule.
- Quantum Mechanical Nature:
- While Rutherford’s model provided a significant advancement, it did not fully account for the quantum mechanical behavior of electrons.
- Later developments, such as quantum mechanics, refined our understanding of atomic structure beyond Rutherford’s classical model.
These key components of Rutherford’s nuclear model revolutionized our understanding of atomic structure and laid the groundwork for further advancements in nuclear physics and quantum mechanics.
Explanation of the central nucleus and surrounding electrons according to Rutherfords Nuclear Model of Atom
Central Nucleus:
- Dense, positively charged core located at the center of the atom.
- Comprises positively charged protons and neutral neutrons.
- Has the majority of the atom’s mass.
- Occupies a tiny volume compared to the overall size of the atom.
- Source of the strong electrostatic attraction that binds electrons to the atom.
Surrounding Electrons:
- Negatively charged particles orbiting the nucleus in specific energy levels or shells.
- Occupy regions of space called orbitals, which represent the probability of finding electrons at various distances from the nucleus.
- Maintain stability by balancing their attraction to the positively charged nucleus with their repulsion from other electrons.
- Move rapidly and continuously around the nucleus in fixed paths, analogous to planets orbiting the sun.
- Electrons in higher energy levels are located farther from the nucleus and possess greater potential energy.
- Electrons can absorb or emit energy in the form of photons when transitioning between energy levels, resulting in the emission or absorption of light.
- Arrangement of electrons in orbitals follows the principles of quantum mechanics, including the aufbau principle, Pauli exclusion principle, and Hund’s rule.
Interaction Between Nucleus and Electrons:
- The positively charged nucleus and negatively charged electrons are held together by Electrostatic Force of Attraction between them.
- Nucleus exerts a force of attraction on electrons, keeping them in orbit around it.
- Electrons experience centripetal force due to the attraction of the nucleus, preventing them from flying off into space.
- The stability of the atom is maintained by the delicate balance between the attractive force of the nucleus and the repulsive forces between electrons.
Rutherford’s nuclear model revolutionized our understanding of atomic structure by proposing a central nucleus surrounded by orbiting electrons. This model provided a more accurate depiction of atomic architecture than previous models and laid the groundwork for further advancements in atomic physics.
Limitations of Rutherfords Nuclear Model of Atom:
- Stability of Electrons:
- Rutherford’s model did not adequately explain why electrons, which are charged particles, do not lose energy and spiral into the nucleus due to electromagnetic radiation.
- According to classical electromagnetism, accelerated charged particles should emit radiation and lose energy, ultimately leading to collapse.
- Quantum Mechanical Behavior:
- Rutherford’s model did not account for the quantized energy levels and discrete electron orbits observed in atomic spectra.
- The model failed to explain phenomena such as spectral lines and the photoelectric effect, which were later elucidated by quantum mechanics.
- Electron Orbits:
- The model suggested that electrons orbit the nucleus in a manner similar to planets orbiting the sun, but this classical analogy contradicts the principles of quantum mechanics.
- Classical physics predicts that orbiting electrons would continuously emit electromagnetic radiation and lose energy, leading to collapse into the nucleus.
- Shell Structure and Periodic Table:
- Rutherford’s model did not provide an explanation for the observed shell structure of electrons and its correlation with the periodic table.
- The model could not account for the filling of electron shells and the periodic trends in atomic properties such as ionization energy and atomic radius.
- Mass Discrepancy:
- The model did not address the observed discrepancy between the mass of the nucleus and the mass of the atom as a whole.
- It did not explain why the nucleus, which contains most of the atom’s mass, occupies such a small volume compared to the overall size of the atom.
- Interaction with Radiation:
- Rutherford’s model did not explain how atoms interact with electromagnetic radiation, such as absorption or emission of photons.
- The model could not account for phenomena such as atomic spectra and the behavior of atoms in electromagnetic fields.
- Relativistic Effects:
- At high speeds or energies, such as those encountered in particle accelerators, relativistic effects become significant and can affect the behavior of electrons and nuclei.
- Rutherford’s model did not incorporate relativistic effects, which are essential for accurately describing atomic and nuclear phenomena at high energies.
While Rutherford’s nuclear model was a significant advancement in atomic theory, it had several limitations that were later addressed by quantum mechanics and relativistic physics. Despite its shortcomings, the model laid the foundation for further developments in understanding the structure and behavior of atoms.
Reflecting on the enduring legacy of Rutherfords Nuclear Model of Atom:
The nuclear model proposed by Ernest Rutherford in 1911 revolutionized our understanding of the atom and left an indelible mark on the field of atomic physics. Despite its limitations, Rutherford’s model laid the foundation for subsequent advancements and continues to influence scientific inquiry to this day.
- Conceptual Shift: Rutherford’s nuclear model represented a paradigm shift in atomic theory. By introducing the concept of a dense, positively charged nucleus surrounded by orbiting electrons, Rutherford challenged the prevailing model of the atom as a homogeneous sphere of positive charge.
- Experimental Validation: The model was supported by experimental evidence, particularly from Rutherford’s own gold foil experiment. The unexpected deflections of alpha particles observed during the experiment provided compelling evidence for the existence of a concentrated nucleus within the atom.
- Quantum Revolution: While Rutherford’s model was based on classical physics, it paved the way for the development of quantum mechanics. Subsequent theoretical advancements, such as the Bohr model and quantum mechanical models, refined our understanding of atomic structure and incorporated principles of quantization and wave-particle duality.
- Technological Applications: The nuclear model contributed to the development of nuclear physics and its applications in fields such as medicine, energy production, and materials science. Understanding the structure and behavior of atomic nuclei has led to advancements in nuclear medicine, radiation therapy, and nuclear energy generation.
- Educational Tool: Rutherford’s nuclear model remains a fundamental concept in science education. It provides a simplified yet valuable framework for teaching students about atomic structure and introduces key concepts such as the nucleus, electron orbitals, and atomic stability.
- Historical Significance: The nuclear model represents a milestone in the history of science. It marked a departure from classical physics and laid the groundwork for modern atomic theory. Rutherford’s contributions to atomic physics earned him widespread recognition, including the Nobel Prize in Chemistry in 1908.
- Inspiration for Further Exploration: While Rutherford’s model had its limitations, it inspired further exploration and inquiry into the nature of the atom. Subsequent generations of scientists built upon his work, leading to deeper insights into atomic and subatomic phenomena.
In conclusion, the enduring legacy of Rutherford’s nuclear model lies not only in its conceptual and experimental contributions to atomic theory but also in its broader impact on science and society. By challenging existing paradigms and inspiring further inquiry, Rutherford’s model continues to shape our understanding of the fundamental building blocks of the universe.
Watch this Video–
Conclusion
In conclusion, Ernest Rutherford’s nuclear model of the atom stands as a testament to the power of scientific inquiry and discovery. Through meticulous experimentation and bold theoretical insight, Rutherford reshaped our understanding of atomic structure and laid the groundwork for modern atomic theory.
Despite its limitations and subsequent refinements by quantum mechanics, Rutherford’s model remains a foundational concept in the study of atomic physics. It provided a crucial stepping stone in the journey toward unraveling the mysteries of the atom and paved the way for a deeper exploration of the quantum realm.
Rutherford’s legacy extends beyond the realm of theoretical physics. His contributions to science have had profound implications for technology, medicine, and our broader understanding of the natural world. From the development of nuclear energy to advancements in medical imaging and cancer treatment, Rutherford’s work continues to impact countless lives.
Moreover, Rutherford’s nuclear model serves as an inspiration for future generations of scientists and thinkers. It demonstrates the importance of questioning prevailing theories, embracing experimentation, and pushing the boundaries of human knowledge.
In a world increasingly shaped by technological innovation and scientific discovery, Rutherford’s nuclear model serves as a reminder of the transformative power of human curiosity and intellect. As we continue to probe the mysteries of the universe, we honor Rutherford’s legacy and strive to build upon his foundational contributions to atomic physics.
FAQs
- What is Rutherfords Nuclear Model of Atom?
- Rutherford’s nuclear model, proposed in 1911, suggests that the atom consists of a dense, positively charged nucleus at its center, surrounded by orbiting electrons.
- What led to the development of Rutherfords Nuclear Model of Atom?
- Rutherford’s model was inspired by the results of his gold foil experiment, where unexpected deflections of alpha particles suggested the presence of a concentrated positive charge within the atom.
- How does Rutherfords Nuclear Model of Atom differ from previous atomic models?
- Unlike previous models, such as Thomson’s plum pudding model, Rutherford’s model proposed that most of the atom’s mass is concentrated in a small, dense nucleus, with electrons orbiting around it.
- What evidence supports Rutherfords Nuclear Model of Atom ?
- The gold foil experiment provided experimental evidence for Rutherford’s model, as the unexpected deflections of alpha particles indicated the presence of a concentrated positive charge within the atom.
- What are the main components of Rutherfords Nuclear Model of Atom?
- The main components include a central nucleus containing positively charged protons and neutral neutrons, surrounded by negatively charged electrons orbiting at a distance.
- What are the limitations of Rutherfords Nuclear Model of Atom?
- Rutherford’s model did not account for the quantized energy levels of electrons observed in atomic spectra, nor did it explain the stability of electron orbits without continuous energy loss.
- How did Rutherfords Nuclear Model of Atom contribute to the development of atomic theory?
- Rutherford’s model represented a significant advancement in atomic theory, providing a more accurate depiction of atomic structure and laying the foundation for further developments in quantum mechanics.
- What are some real-world applications of Rutherfords Nuclear Model of Atom?
- Rutherford’s model has applications in various fields, including nuclear medicine, energy production, and materials science, where understanding atomic structure is crucial.
- Was Rutherfords Nuclear Model of Atom completely accurate?
- While Rutherford’s model laid the groundwork for modern atomic theory, subsequent advancements in quantum mechanics refined our understanding of atomic structure beyond the classical model.
- What is the legacy of Rutherfords Nuclear Model of Atom in the history of science?
- Rutherford’s nuclear model represents a landmark achievement in the history of science, demonstrating the power of experimentation and theoretical insight in advancing our understanding of the universe.
Also Read-
https://pcmconceptclear.com/best-book-for-science-class-11-cbse/
https://pcmconceptclear.com/best-science-book-for-class-11-isc/
https://pcmconceptclear.com/some-basic-concepts-of-chemistry/
https://pcmconceptclear.com/mole-concept-iit-jee-pyqs-with-solutions/
https://pcmconceptclear.com/mastering-mole-concepts-class-11/
https://pcmconceptclear.com/some-basic-concepts-of-chemistry-cbse-11/
https://pcmconceptclear.com/acids-bases-salts-most-imp-qa/
https://pcmconceptclear.com/chemical-reactions-and-equations-class-10-science/
https://pcmconceptclear.com/electricity-class-10-made-easy/
7 thoughts on “Rutherfords Nuclear Model of Atom and Its Impact: Chem11(2025)”