Importance of Understanding the Classification of Elements in Chemistry
Organizes Chemical Knowledge
- Simplifies the study of elements by grouping them based on similar properties.
- Helps in predicting the behavior of elements in reactions.
Foundation for Advanced Studies
- Provides a basis for studying complex chemical theories and reactions.
- Essential for understanding topics like chemical bonding, stoichiometry, and thermodynamics.
Identifies Trends and Patterns
- Reveals trends in properties such as atomic radius, ionization energy, and electronegativity.
- Helps in making sense of periodic trends and the behavior of elements
Early Attempts for Classification of Elements
Lavoisier’s List of Elements (1789)
Lavoisier compiled a list of 33 elements in which he tried to distinguish between metals and non-metals.
His list was one of the first systematic attempts to categorize elements.
De Chancourtois’ Telluric Helix (1862)
Alexandre-Émile Béguyer de Chancourtois created a three-dimensional arrangement of elements in a helical form.
He arranged elements by increasing atomic weight, which highlighted periodic properties in a spiral on a cylinder.
Lothar Meyer’s Graphs (1864)
Lothar Meyer plotted graphs of atomic volume against atomic weight, showing periodic trends.
His work independently demonstrated the periodicity of elemental properties, similar to Mendeleev’s findings.
Doebereiner’s Triads
| Element | Atomic Weight | Element | Atomic Weight | Element | Atomic Weight |
|---|---|---|---|---|---|
| Li | 7 | Ca | 40 | Cl | 35.5 |
| Na | 23 | Sr | 88 | Br | 80 |
| K | 39 | Ba | 137 | I | 127 |
Introduction to Dobereiner’s Triads
Proposed by Johann Wolfgang Döbereiner in 1829.
An early attempt to classify elements based on their properties.
Concept of Triads
Elements were grouped into sets of three, called triads.
Each triad consisted of elements with similar chemical properties.
Atomic Weight Relationship
In each triad, the atomic weight of the middle element was approximately the average of the atomic weights of the other two elements.
Example: The triad of calcium (Ca), strontium (Sr), and barium (Ba).
-
-
- Calcium: Atomic weight = 40
- Strontium: Atomic weight = 88
- Barium: Atomic weight = 137
- Average of Ca and Ba: (40 + 137) / 2 = 88.5 (close to Sr’s atomic weight)
-
Examples of Triads
Alkali Metals Triad:
-
-
- Lithium (Li), Sodium (Na), Potassium (K)
-
Halogens Triad:
-
-
- Chlorine (Cl), Bromine (Br), Iodine (I)
-
Significance of Dobereiner’s Triads in Classification of Elements
Highlighted the existence of relationships between elements.
A foundation was provided for the development of the periodic table.
Showed that elements could be grouped based on shared properties and atomic weights.
Encouraged further research into the systematic classification of elements.
Limitations of Dobereiner’s Triads in the Classification of Elements
All elements could not be grouped into triads.
Only a limited number of triads were identified, which restricted its applicability.
Newlands’ Law of Octaves
Introduction to Newlands’ Law of Octaves
Proposed by John Newlands in 1864.
An early attempt to classify elements based on their atomic weights.
Concept of Octaves
Newlands’ Octaves
| Element | Li | Be | B | C | N | O | F |
|---|---|---|---|---|---|---|---|
| At. wt. | 7 | 9 | 11 | 12 | 14 | 16 | 19 |
| Element | Na | Mg | Al | Si | P | S | Cl |
|---|---|---|---|---|---|---|---|
| At. wt. | 23 | 24 | 27 | 29 | 31 | 32 | 35.5 |
| Element | K | Ca | |||||
|---|---|---|---|---|---|---|---|
| At. wt. | 39 | 40 |
With this concept, elements were arranged in order of increasing atomic weight.
Observed that every eighth element had properties similar to the first, similar to the musical octave.
Arrangement of Elements
Elements were listed in rows of seven, with the eighth element starting a new row.
Example:
Li (Lithium) ,
Be (Beryllium),
B (Boron),
C (Carbon),
N (Nitrogen),
O (Oxygen),
F (Fluorine),
Na (Sodium),
(eighth element, similar to Lithium)
Periodic Pattern
Noted a repeating or periodic pattern in properties every eighth element.
Helped in recognizing the periodicity in the properties of elements.
Limitations of Newlands’ Law of Octaves in the Classification of Elements
Applicability to Light Elements
The law was only applicable to elements with low atomic weights.
Failed to accommodate elements beyond calcium.
Inconsistencies
Elements with dissimilar properties were forced into the same groups.
Example: Iron (Fe) was grouped with elements like oxygen (O) and sulfur (S).
Impact of Newlands’ Law of Octaves in the Classification of Elements
Despite its limitations, Newlands’ Law of Octaves was significant in highlighting the periodicity of elements.
Paved the way for the development of more comprehensive periodic tables, such as Mendeleev’s periodic table.
Best Reference Book for CBSE Chemistry11
Click the Amazon Link To Buy/Check – https://amzn.to/4kPzwyi
Pradeep’s New Course Chemistry for Class 11 (Vol. 1 & 2) Examination 2025-26

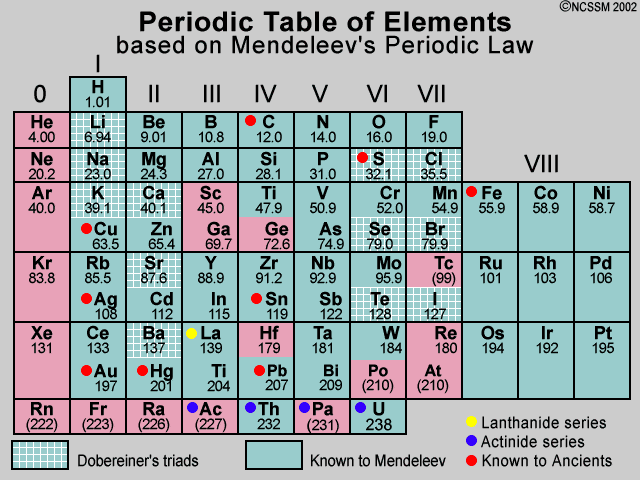
Mendeleev’s Periodic Table

How Mendeleev Organized the Classification of Elements
Arrangement by Atomic Weight
Mendeleev arranged the elements in order of increasing atomic weight.
similar properties appeared at regular intervals.
Formation of Periods and Groups
Rows in the table were called periods, and columns were called groups.
Elements in the same group had similar chemical properties.
Mendeleev’s Periodic Law
Formulated the periodic law: The properties of elements are a periodic function of their atomic weights.
This law explained the repeating patterns of element properties.
Predictive Gaps
-
- Gaps were left in his table for elements that had not yet been discovered.
- Accurately predicted properties of elements like gallium (Ga) and germanium (Ge) before they were discovered.
Adjustment for Anomalies
-
- Made adjustments for elements that did not fit the pattern strictly by atomic weight.
- For example, he placed iodine (I) after tellurium (Te) despite its lower atomic weight, based on properties.
Grouping of Similar Elements
-
- Elements with similar properties were grouped together.
- For example, alkali metals (Li, Na, K) were placed in the same group due to their similar reactivity.
Correction of Atomic Weights
-
- Mendeleev’s table led to the correction of some atomic weights.
- Helped to standardize atomic weights based on his periodic law.
Impact on Chemistry
-
- Mendeleev’s periodic table significantly advanced the understanding of elemental properties.
- Provided a powerful tool for chemists to predict chemical behavior and discover new elements.
Gaps for Undiscovered Elements in Mendeleev’s Classification of Elements
Intentional Gaps in the Table
-
- Empty spaces were left by Mendeleev in his periodic table.
- These gaps were for elements that had not yet been discovered.
Logical Placement
-
- The gaps were placed based on observed periodic trends and properties.
- Mendeleev predicted where these elements would fit in the table.
Prediction of Existence
-
- Mendeleev believed that these undiscovered elements would eventually be found.
- His table provided a framework for where to look for new elements.
Examples of Gaps
-
- Notable gaps included those for elements like gallium (Ga) and germanium (Ge).
- These elements were later discovered and fit into the predicted gaps perfectly.
Prediction of Properties of Missing Elements in Mendeleev’s Classification of Elements
Use of Periodic Trends
-
- Mendeleev used known properties of surrounding elements to predict the properties of missing elements.
- He compared elements within the same group and period to make his predictions.
Prediction of Physical Properties
-
- Predicted properties such as atomic weight, density, and melting point.
- Example: Predicted the atomic weight of gallium (Ga) to be around 68; the actual value is 69.7.
Prediction of Chemical Properties
-
- Anticipated chemical behavior and reactivity.
- Example: Predicted that gallium (Ga) would form compounds similar to those of aluminum (Al).
Naming of Predicted Elements
-
- Used prefix “eka-” (meaning “one” in Sanskrit) to name the missing elements.
- Example: Predicted gallium was referred to as “eka-aluminum.”
Validation of Predictions
-
- The eventual discovery of these elements confirmed Mendeleev’s predictions.
- Properties of discovered elements closely matched his forecasts.
Examples of Predicted Elements
-
- Gallium (Ga): Predicted as eka-aluminum.
- Germanium (Ge): Predicted as eka-silicon.
- Scandium (Sc): Predicted as eka-boron.
Contributions of Mendeleev’s Table in the Classification of Elements
Systematic Organization
-
- Mendeleev’s table organized elements in a logical order based on atomic weight.
- Grouped elements with similar properties together.
Prediction of New Elements
-
- Gaps were left for undiscovered elements, predicting their existence and properties.
- Encouraged the discovery of elements like gallium (Ga) and germanium (Ge).
Foundation for Modern Periodic Table
-
- Mendeleev’s work laid the foundation for the modern periodic table.
- Inspired later scientists to refine and improve the periodic classification of elements.
Identification of Periodic Trends
-
- Recognized trends in properties such as atomic radius, ionization energy, and electronegativity.
- Helped in understanding the periodic behavior of elements.
Limitations of Mendeleev’s Periodic Table in the Classification of Elements
Atomic Weight-Based Arrangement
-
- Mendeleev’s table was based on atomic weight, not atomic number.
- Some elements did not fit well when arranged strictly by atomic weight.
Position of Isotopes
-
- Did not account for isotopes (elements with the same atomic number but different atomic weights).
- Led to inconsistencies in the placement of elements.
Anomalous Pairs
-
- Some pairs of elements (like Te and I) had to be placed out of atomic weight order to fit properties.
- This created anomalies and exceptions in the table.
Lack of Group Clarification
-
- Some groups, especially transition metals, were not clearly defined.
- The table lacked the clear categorization seen in the modern periodic table.
Inability to Explain All Properties
-
- Could not explain the underlying reasons for periodic trends and elemental properties.
- Lacked the theoretical foundation provided by later quantum mechanics.
Placement of Hydrogen
-
- The position of hydrogen remained ambiguous.
- Hydrogen’s properties did not fit neatly into any one group.
No Separate Block for Noble Gases
-
- Noble gases were not included initially as they were discovered later.
- The modern periodic table includes a separate group for noble gases, which Mendeleev’s table did not.
Modern Periodic Table for Classification of Elements

Transition from Mendeleev’s Table to the Modern Periodic Table
- Mendeleev’s Initial Framework
- Dmitri Mendeleev arranged elements by increasing atomic weight and similar properties.
- Left gaps for undiscovered elements and accurately predicted their properties.
- Discovery of New Elements
- New elements discovered filled the gaps in Mendeleev’s table.
- Validated Mendeleev’s predictions and reinforced the periodic law.
- Identification of Atomic Number
- Henry Moseley (1913) determined that atomic number, not atomic weight, is the fundamental property.
- Moseley’s experiments with X-ray spectra showed that elements should be arranged by atomic number.
- Modern Periodic Law
- It states that properties of elements are a periodic function of their atomic numbers.
- Replaced Mendeleev’s atomic weight-based arrangement.
- Rearrangement of Elements
- Now, the elements were rearranged in order of increasing atomic number.
- Resolved inconsistencies such as the placement of iodine (I) and tellurium (Te).
- Recognition of Periodic Trends
- Clear trends and periodicity emerged when elements were ordered by atomic number.
- Trends in atomic radius, ionization energy, and electronegativity became more apparent.
- Introduction of Noble Gases
- Noble gases were discovered and added as a new group (Group 18) in the periodic table.
- Provided a complete understanding of the periodicity of elements.
- Development of Electron Configuration Theory
- Quantum mechanics and electron configuration theory explained the periodic trends observed.
- Elements were grouped based on their electron configurations (s, p, d, f blocks).
- Modern Periodic Table Structure
- Modern table is organized into periods (rows) and groups (columns).
- Elements with similar properties are in the same group, reflecting their similar electron configurations.
- Periodic Table Adaptations
- The table has been expanded to include synthetic elements.
- Lanthanides and actinides were placed separately to keep the table concise.
Explanation of the Modern Periodic Law Based on Atomic Number
- Definition of Modern Periodic Law
- This law states that the properties of elements are a periodic function of their atomic numbers.
- Atomic Number Concept
- The identity of the element and its position in the periodic table are determined by it.
- Arrangement by Atomic Number
- In modern Period Table, elements are arranged in increasing order of atomic number.
- This arrangement places elements with similar properties in the same groups (columns).
- Periodic Trends
- When arranged in the increasing order of atomic number, properties of elements repeat periodically.
- Examples include trends in atomic radius, ionization energy, and electronegativity.
- Grouping of Elements
- Elements in the same group have similar chemical and physical properties due to their similar electron configurations.
- For example, alkali metals (Group 1) are highly reactive and have similar properties.
- Periodicity in Properties
- As you move across a period (row) from left to right, elements show a gradual change in properties.
- For example, atomic radius decreases, and ionization energy increases across a period.
- Resolution of Anomalies
- The atomic number-based arrangement resolved inconsistencies seen in Mendeleev’s table, such as the placement of iodine and tellurium.
- Ensured that elements with similar properties are grouped together.
- Electron Configuration
- The modern periodic table reflects the arrangement of electrons in atomic orbitals (s, p, d, f blocks).
- Elements are classified based on their electron configurations, which explain periodic properties.
Classification of Elements in the Modern Periodic Table
Explanation of Groups (Vertical Columns)
Definition of Groups
Groups are the vertical columns in the periodic table.
There are 18 groups in the modern periodic table.
Similar Chemical Properties
Elements in the same group have similar chemical properties.
This similarity is due to having the same number of valence electrons.
Group Classification
Groups are numbered from 1 to 18.
Group number indicates the number of valence electrons.
For example, Group 1 elements have 1 valence electron, and Group 17 elements have 7 valence electrons.
Groups 1 and 2 are the s-block elements, while Groups 13 to 18 are the p-block elements.
Reactivity Trends
Reactivity often increases down the group for metals and decreases down the group for nonmetals.
Example: Alkali metals (Group 1) become more reactive as you move down the group, while halogens (Group 17) become less reactive.
Common Groups
Group 1: Alkali Metals – Highly reactive metals including lithium (Li), sodium (Na), and potassium (K).
Group 2: Alkaline Earth Metals – Reactive metals including beryllium (Be), magnesium (Mg), and calcium (Ca).
Group 17: Halogens – Reactive nonmetals including fluorine (F), chlorine (Cl), and bromine (Br).
Group 18: Noble Gases – Inert gases including helium (He), neon (Ne), and argon (Ar).
Explanation of Periods (Horizontal Rows)
Definition of Periods
Periods are the horizontal rows in the periodic table.
There are seven periods in the modern periodic table.
Trend Across Periods
As you move from left to right across a period, the atomic number increases.
Each element has one more proton and electron than the element before it.
Properties of elements change gradually across a period, such as decreasing atomic radius and increasing ionization energy.
Electron Configuration
Elements in the same period have electrons filling the same principal energy level or shell.
Example: In Period 2, elements like lithium (Li) and neon (Ne) have electrons in the second energy level.
Electrons are added to the same principal energy level as you move across the period.
Period Length
The length of a period corresponds to the number of elements filling that energy level.
The number of elements in a period depends on the number of available electron orbitals.
Period 1 has 2 elements, while periods 2 and 3 have 8 elements each. Periods 4 and 5 have 18 elements each, and periods 6 and 7 can have up to 32 elements each.
Atomic Radius: Decreases across a period due to increasing positive charge in the nucleus pulling electrons closer.
Ionization Energy: Increases across a period as the nucleus holds electrons more tightly.
Electronegativity: Increases across a period, reflecting the tendency to attract electrons.
Understanding the classification of elements into groups and periods helps in predicting their properties and behaviors, making the periodic table a valuable tool in chemistry.
Description of Blocks
s-Block Elements
Electron Configuration: Last electrons added to the s-orbital.
Their general electron configuration is=
![]()
Position in Periodic Table
Located in Groups 1 and 2 of the periodic table.
Group 1 is known as the alkali metals, and Group 2 is known as the alkaline earth metals.
Properties:
Includes alkali metals (Group 1) and alkaline earth metals (Group 2). They are typically highly reactive and have low ionization energies.
Low Density
Group 1 Elements: Generally have low densities (e.g., lithium, sodium, potassium float on water).
Group 2 Elements: Have higher densities compared to Group 1 elements but are still relatively low compared to many other metals.
High Reactivity
Group 1 Elements: Highly reactive, especially with water, forming hydroxides and releasing hydrogen gas.
Group 2 Elements: Less reactive than Group 1 but still react with water and acids to form hydroxides and salts.
Formation of Basic Oxides
Group 1 Elements: Form strong bases (alkalies) when they react with water, producing hydroxides (e.g., sodium hydroxide).
Group 2 Elements: Also form basic oxides and hydroxides but are less soluble in water compared to Group 1 hydroxides.
Examples
Group 1: Alkali Metals
-
- Lithium (Li): Used in rechargeable batteries and some medications.
- Sodium (Na): Common in table salt (sodium chloride) and streetlights.
- Potassium (K): Important for biological functions and fertilizers.
Group 2: Alkaline Earth Metals
-
- Beryllium (Be): Used in aerospace materials and X-ray windows.
- Magnesium (Mg): Used in lightweight alloys and as a component in fireworks.
- Calcium (Ca): Essential for bone health and used in cement and lime.
p-Block Elements
Electron Configuration: Last electrons added to the p-orbital.
General Outer Shell Electronic Configuration of p-Block elements-
![]()
Position in Periodic Table
-
- Located in Groups 13 to 18 of the periodic table.
Diverse Properties
-
- Includes metals, metalloids, and nonmetals.
- Properties vary widely, from highly reactive nonmetals to less reactive metals.
Variable Reactivity
-
- Nonmetals (e.g., halogens) are highly reactive.
- Noble gases are largely unreactive.
- Metalloids and metals have variable reactivity.
Oxidation States
-
- Elements exhibit multiple oxidation states.
- This leads to a wide range of chemical compounds and reactivity patterns.
Formation of Compounds
-
- Nonmetals form covalent compounds.
- Metals form ionic compounds.
- Metalloids can exhibit properties of both metals and nonmetals.
Physical States
-
- p-Block elements exist in all three physical states at room temperature: gases (e.g., nitrogen, oxygen), liquids (e.g., bromine), and solids (e.g., carbon, silicon).
Variable Metallic Character
-
- Metallic character increases down the group and decreases across the period.
- Metals (e.g., aluminum), metalloids (e.g., silicon), and nonmetals (e.g., sulfur) are all found in the p-block.
Diverse Applications
-
- Used in a wide range of applications, from everyday items to advanced technology.
- Examples include semiconductors, fertilizers, and inert atmospheres.
Examples
- Group 13: Boron Group
- Boron (B): Used in glassmaking and detergents.
- Aluminum (Al): Used in packaging, construction, and aircraft.
- Group 14: Carbon Group
- Carbon (C): Found in all organic life, used in fuels and materials like graphite and diamond.
- Silicon (Si): Essential in electronics as a semiconductor and used in solar panels.
- Group 15: Nitrogen Group
- Nitrogen (N): Makes up 78% of the Earth’s atmosphere, used in fertilizers and explosives.
- Phosphorus (P): Used in fertilizers, detergents, and DNA structure.
- Group 16: Oxygen Group (Chalcogens)
- Oxygen (O): Essential for respiration, used in steelmaking and water treatment.
- Sulfur (S): Used in the production of sulfuric acid, fertilizers, and matches.
- Group 17: Halogens
- Fluorine (F): Used in toothpaste, Teflon, and refrigerants.
- Chlorine (Cl): Used in water disinfection, bleach, and PVC production.
- Group 18: Noble Gases
- Helium (He): Used in balloons, as a cooling agent, and in cryogenics.
- Neon (Ne): Used in neon signs and high-voltage indicators.
d-Block Elements
Electron Configuration: Last electrons added to the d-orbital.
General Outer Shell Electronic Configuration of d-Block elements-
![]()
Position in Periodic Table
Located in Groups 3 to 12 of the periodic table.
Variable Oxidation States
Exhibit multiple oxidation states due to the involvement of d-electrons in bonding.
Common oxidation states include +2 and +3, but can range from -2 to +7.
Formation of Colored Compounds
Many d-block elements form colored compounds.
The colors arise from electronic transitions between d-orbital energy levels.
Catalytic Properties
Many transition metals and their compounds act as catalysts in chemical reactions.
Examples include iron in the Haber process and nickel in hydrogenation reactions.
Magnetic Properties
Exhibit magnetic properties due to unpaired d-electrons.
Examples include ferromagnetism in iron, cobalt, and nickel.
High Melting and Boiling Points
Generally have high melting and boiling points.
Due to strong metallic bonding involving d-electrons.
Formation of Complexes
Transition metals form complex ions with ligands.
This involves coordination bonds between the metal ion and ligands (molecules or ions).
Conductivity
Good conductors of heat and electricity.
Due to the presence of free-moving d-electrons.
Dense and Hard
Transition metals are typically dense and hard.
They have a closely packed atomic structure.
Examples
Iron (Fe)
Used in construction (steel), manufacturing, and as a catalyst in the Haber process.
Exhibits oxidation states of +2 and +3.
Copper (Cu)
Used in electrical wiring, coins, and plumbing.
Exhibits oxidation states of +1 and +2.
Forms colored compounds like blue copper(II) sulfate.
Zinc (Zn)
Used in galvanization to protect iron from rusting, in alloys like brass, and in batteries.
Exhibits an oxidation state of +2.
Silver (Ag)
Used in jewelry, silverware, photography, and electronics.
Exhibits an oxidation state of +1.
Known for its high electrical conductivity.
Gold (Au)
Used in jewelry, electronics, and as a monetary standard.
Exhibits oxidation states of +1 and +3.
Known for its resistance to corrosion and tarnish.
Platinum (Pt)
Used in catalytic converters, jewelry, and as a catalyst in various chemical reactions.
Exhibits oxidation states of +2 and +4.
Highly resistant to corrosion.
Chromium (Cr)
Used in stainless steel, chrome plating, and pigments.
Exhibits oxidation states of +2, +3, and +6.
Forms colorful compounds like red chromium(III) oxide.
Properties: These metals are characterized by their ability to form colored compounds, variable oxidation states, and magnetic properties.
f-Block Elements
Electron Configuration
f-Block elements have their last electron added to an f-orbital.
General Outer Shell Electronic Configuration of f-Block elements-

![]()
Position in Periodic Table
-
- Located in two rows at the bottom of the periodic table: the lanthanides and actinides.
Lanthanides
-
- Elements from atomic number 57 (lanthanum) to 71 (lutetium).
- Known as rare earth elements.
Actinides
-
- Elements from atomic number 89 (actinium) to 103 (lawrencium).
- Many are radioactive.
Variable Oxidation States
-
- Exhibit multiple oxidation states, commonly +3.
- Actinides have a greater range of oxidation states compared to lanthanides.
Formation of Colored Ions
-
- Many f-block elements form colored ions.
- The colors result from electronic transitions within the f-orbitals.
Magnetic Properties
-
- Exhibit magnetic properties due to unpaired f-electrons.
- Examples include neodymium and gadolinium.
High Density and High Melting Points
-
- Generally have high density and high melting points.
- Due to strong metallic bonding involving f-electrons.
Radioactivity
-
- Many actinides are radioactive.
- Uranium and thorium are well-known examples.
Formation of Complexes
-
- f-Block elements readily form complex ions with various ligands.
- Involves coordination bonds between the metal ion and ligands.
Examples
Lanthanides
-
- Cerium (Ce)
- Used in catalytic converters and in the glass industry.
- Exhibits oxidation states of +3 and +4.
- Neodymium (Nd)
- Used in strong permanent magnets (neodymium magnets).
- Exhibits oxidation state of +3.
- Gadolinium (Gd)
- Used in MRI contrast agents and in nuclear reactors.
- Exhibits oxidation state of +3.
- Cerium (Ce)
Actinides
-
- Uranium (U)
- Used as a fuel in nuclear reactors and in nuclear weapons.
- Exhibits oxidation states of +3, +4, +5, and +6.
- Thorium (Th)
- Used as a nuclear fuel and in gas mantles.
- Exhibits oxidation states of +3 and +4.
- Plutonium (Pu)
- Used in nuclear reactors and nuclear weapons.
- Exhibits oxidation states of +3, +4, +5, and +6.
- Uranium (U)
Properties: Includes rare earth elements (lanthanides) and actinides, many of which are radioactive. They have unique properties like high magnetic susceptibility and are often used in advanced materials.
Trends in the Modern Periodic Table
Trend of Atomic Radius
Across Periods
- Definition
- Atomic radius is the distance from the nucleus to the outermost shell of an electron.
- Trend Across a Period
- Atomic radius decreases from left to right across a period.
- Reason for Decrease
- As you move across a period, the number of protons in the nucleus increases.
- Increased nuclear charge pulls electrons closer to the nucleus.
- Electrons are added to the same energy level, without additional shielding effect.
- Example
- In Period 2: Lithium (Li) has a larger atomic radius than Fluorine (F).
- In Period 3: Sodium (Na) has a larger atomic radius than Chlorine (Cl).
Down Groups
- Trend Down a Group
- Atomic radius increases from top to bottom within a group.
- Reason for Increase
- As you move down a group, additional electron shells are added.
- Increased number of shells means outer electrons are further from the nucleus.
- Inner electron shells provide shielding, reducing the effective nuclear charge felt by outer electrons.
- Example
- In Group 1: Lithium (Li) has a smaller atomic radius than Cesium (Cs).
- In Group 17: Fluorine (F) has a smaller atomic radius than Iodine (I).
Variation of Ionization Energy
Across Periods
- Definition
- Ionization energy is the energy required to remove an electron from a gaseous atom or ion.
- Trend Across a Period
- Ionization energy generally increases from left to right across a period.
- Reason for Increase
- As you move across a period, the nuclear charge increases.
- Increased nuclear charge attracts electrons more strongly, making them harder to remove.
- Atomic radius decreases across a period, so electrons are closer to the nucleus and more strongly attracted.
- Example
- In Period 2: Lithium (Li) has a lower ionization energy than Fluorine (F).
- In Period 3: Sodium (Na) has a lower ionization energy than Chlorine (Cl).
- Exceptions
- Small drops in ionization energy can occur between Group 2 and Group 13, and Group 15 and Group 16, due to electron configuration and subshell arrangements.
Down Groups
- Trend Down a Group
- Ionization energy generally decreases from top to bottom within a group.
- Reason for Decrease
- As you move down a group, additional electron shells are added.
- Increased distance between the nucleus and the outermost electrons reduces the nuclear attraction.
- Increased shielding effect from inner electron shells reduces the effective nuclear charge felt by outer electrons.
- Example
- In Group 1: Lithium (Li) has a higher ionization energy than Cesium (Cs).
- In Group 17: Fluorine (F) has a higher ionization energy than Iodine (I).
- Shielding Effect
- The inner shells of electrons shield the outer electrons from the full effect of the nuclear charge.
- This makes it easier to remove outer electrons as you go down a group.
Trend of Electronegativity
Definition
- Electronegativity: The ability of an atom to attract electrons towards itself in a chemical bond.
Across Periods
- Trend Across a Period
- Electronegativity increases from left to right across a period.
- Reason for Increase
- Increased Nuclear Charge: As the number of protons in the nucleus increases, the nucleus attracts bonding electrons more strongly.
- Decreased Atomic Radius: Electrons are closer to the nucleus, enhancing the nucleus’s ability to attract bonding electrons.
- Example
- In Period 2: Lithium (Li) has a lower electronegativity than Fluorine (F).
- In Period 3: Sodium (Na) has a lower electronegativity than Chlorine (Cl).
Down Groups
- Trend Down a Group
- Electronegativity decreases from top to bottom within a group.
- Reason for Decrease
- Increased Atomic Radius: As additional electron shells are added, the bonding electrons are farther from the nucleus.
- Increased Shielding Effect: Inner electrons shield the outer electrons from the nucleus’s pull, reducing the nucleus’s ability to attract bonding electrons.
- Example
- In Group 1: Lithium (Li) has a higher electronegativity than Cesium (Cs).
- In Group 17: Fluorine (F) has a higher electronegativity than Iodine (I).
General Observations
- Most Electronegative Element
- Fluorine (F) is the most electronegative element in the periodic table.
- Least Electronegative Elements
- Alkali metals (Group 1) and alkaline earth metals (Group 2) have the lowest electronegativities.
Explanation and Trend of Electron Affinity
Definition
- Electron Affinity: The amount of energy released when an atom in the gaseous state accepts an electron to form a negative ion.
Across Periods
- Trend Across a Period
- Electron affinity generally increases from left to right across a period.
- Reason for Increase
- Increased Nuclear Charge: As the number of protons in the nucleus increases, the nucleus attracts additional electrons more strongly.
- Decreased Atomic Radius: Electrons added to atoms with smaller radii are closer to the nucleus, leading to a stronger attraction and greater energy release.
- Example
- In Period 2: Lithium (Li) has a lower electron affinity than Fluorine (F).
- In Period 3: Sodium (Na) has a lower electron affinity than Chlorine (Cl).
- Exceptions
- Some elements, such as noble gases and some Group 2 and Group 15 elements, have low or positive electron affinities due to stable electron configurations.
Down Groups
- Trend Down a Group
- Electron affinity generally decreases from top to bottom within a group.
- Reason for Decrease
- Increased Atomic Radius: As additional electron shells are added, the added electron is further from the nucleus, leading to a weaker attraction and less energy release.
- Increased Shielding Effect: Inner electron shells shield the added electron from the full effect of the nuclear charge, reducing the attraction and energy release.
- Example
- In Group 1: Lithium (Li) has a higher electron affinity than Cesium (Cs).
- In Group 17: Chlorine (Cl) has a higher electron affinity than Iodine (I).
General Observations
- High Electron Affinity
- Halogens (Group 17) have high electron affinities, with Chlorine (Cl) often cited as having the highest electron affinity.
- Low Electron Affinity
- Noble gases (Group 18), alkali metals (Group 1), and alkaline earth metals (Group 2) have low or even positive electron affinities, indicating reluctance to gain electrons.
Understanding these trends in electron affinity helps in predicting how atoms will interact when forming ions and contributes to the broader understanding of chemical reactivity and bonding.
Conclusion
Understanding the classification of elements is not just about memorizing the periodic table, but about appreciating the underlying principles that govern the behavior of matter, which is crucial for success in future chemistry studies and related fields.
WATCH THIS VIDEO-
ALSO READ-
https://pcmconceptclear.com/best-book-for-science-class-11-cbse/
https://pcmconceptclear.com/best-science-book-for-class-11-isc/
https://pcmconceptclear.com/chemistry-class-11-structure-of-atom/
https://pcmconceptclear.com/some-basic-concepts-of-chemistry/
https://pcmconceptclear.com/mastering-mole-concepts-class-11/
https://pcmconceptclear.com/some-basic-concepts-of-chemistry-cbse-11/
3 thoughts on “Classification of Elements: Chemistry Class 11(2025-26)”